Have you ever pondered the three-dimensional shape of a molecule, wondering how its atoms are arranged in space? This intricate dance of atoms, dictating a molecule’s properties and reactivity, is the realm of VSEPR theory. VSEPR stands for Valence Shell Electron Pair Repulsion theory, and it provides a framework for predicting the geometry of molecules based on the repulsion of electron pairs around a central atom.

Image: studylib.net
This article will delve into the world of VSEPR practice problems, equipping you with the tools to predict molecular geometries, understand the reasoning behind them, and even create your own practice problems. We’ll guide you through the process, exploring various examples, and providing a valuable PDF resource with answers to reinforce your learning journey.
Understanding the Basics of VSEPR Theory
Imagine a central atom surrounded by electron pairs, like a group of magnets jostling for position. These electron pairs, encompassing both bonding and lone pairs, repel each other, seeking maximum distance to minimize electrostatic repulsion. This interplay of repulsion drives the molecule to adopt a specific shape, a key aspect in determining its properties.
Key Concepts for Success
To master VSEPR problems, we need to grasp some fundamental concepts:
- Electron Domains: A region around a central atom where electrons reside, either in bonding or non-bonding pairs.
- Lone Pair: Non-bonding electron pair residing on the central atom.
- Bonding Pair: Pair of electrons shared between two atoms, forming a covalent bond.
- Steric Number: Total number of electron domains surrounding the central atom.
VSEPR Practice Problems: A Step-by-Step Approach
Let’s dive into the exciting world of VSEPR practice problems. Each problem involves a molecule, and your mission is to predict its geometry. We’ll break down the process into clear steps:
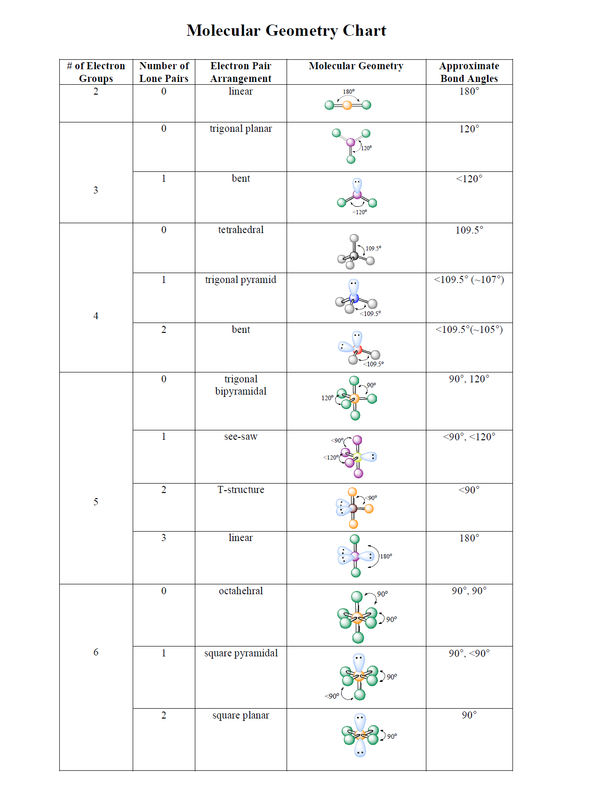
Image: mmstcchemistry.weebly.com
Step 1: Draw the Lewis Structure
This crucial step involves determining the arrangement of atoms and lone pairs in the molecule. Consider the following example: Water (H2O).
1. Count the total number of valence electrons: H (1) x 2 + O (6) = 8 valence electrons
2. Connect the atoms with single bonds: H-O-H
3. Distribute the remaining electrons to achieve octets (or duets for hydrogen) for each atom:
H
|
:O:
|
H
Step 2: Determine the Steric Number
For water, the central oxygen atom has two bonding pairs and two lone pairs, resulting in a steric number of 4 (2 + 2 = 4). This number indicates the total number of electron domains around the central atom.
Step 3: Predict the Molecular Geometry
Using the steric number and the VSEPR model, we can predict the geometry. With a steric number of 4, water adopts a tetrahedral electron domain geometry. However, since it has two lone pairs, the molecular geometry is actually bent or angular.
Step 4: Use VSEPR Diagram
Visual aids like VSEPR diagrams can help visualize the geometry. These diagrams depict the central atom with surrounding electron domains, represented by lines or dots. The lone pairs are typically represented by dots.
Step 5: Consider Bond Angles
The bond angles are affected by the repulsion between electron domains. For example, in water, the bond angle is slightly less than 109.5 degrees, the ideal angle in a tetrahedral geometry, because the lone pairs exert a stronger repulsion than bonding pairs.
VSEPR Practice Problems: A Variety of Examples
Let’s practice with a couple of additional examples:
Example 1: Carbon Dioxide (CO2)
1. Lewis Structure: O=C=O
2. Steric Number: 2 (two double bonds)
3. Molecular Geometry: Linear
4. Bond Angle: 180 degrees
Example 2: Ammonia (NH3)
1. Lewis Structure:
H
|
:N:
|
H
|
H
2. Steric Number: 4 (three bonding pairs and one lone pair)
3. Molecular Geometry: Trigonal pyramidal
4. Bond Angle:Slightly less than 109.5 degrees
VSEPR Practice Problems PDF: A Powerful Resource
To master VSEPR theory, hands-on practice is essential. That’s why we’ve created a valuable resource for you: a PDF containing a wide variety of VSEPR practice problems with detailed solutions. This resource will allow you to tackle problems independently and self-assess your progress.
The PDF will cover a wide range of molecules, ranging from simple ones like ammonia to more complex structures, helping you develop a comprehensive understanding of VSEPR theory.
To access this PDF, you can download it from the link below:
[Insert Link to PDF Here]
Exploring Real-World Applications of VSEPR
VSEPR theory isn’t just a theoretical concept; it has far-reaching applications in various fields:
- Chemistry: Predicting the shape and properties of molecules, understanding their reactions, and designing new compounds.
- Biochemistry: Elucidating the structure of proteins and enzymes, essential for biological processes.
- Materials Science: Designing materials with specific properties based on their molecular geometry.
- Nanotechnology: Understanding the behavior of molecules at the nanoscale level.
VSEPR theory’s impact extends beyond the laboratory, influencing fields like drug discovery, environmental management, and energy production.
Vsepr Practice Problems With Answers Pdf
Conclusion
Mastering VSEPR theory is a fundamental step in understanding the fascinating world of chemistry. By understanding the principles behind molecular geometry and practicing with a range of problems, you can unlock a deeper appreciation for how atoms arrange themselves to create the intricate tapestry of the world around us. We encourage you to dive into the PDF resource, explore additional problems online, and continue your journey of scientific discovery.






