Ever wondered how nutrients reach every corner of your body or how waste products are efficiently removed? It all boils down to the fascinating dance of molecules, driven by the principles of diffusion and osmosis. These cellular processes, seemingly invisible to the naked eye, are the lifeblood of every living organism. Today, we embark on a journey to demystify these fundamental concepts by dissecting the answers to Lab 1’s exploration of diffusion and osmosis.
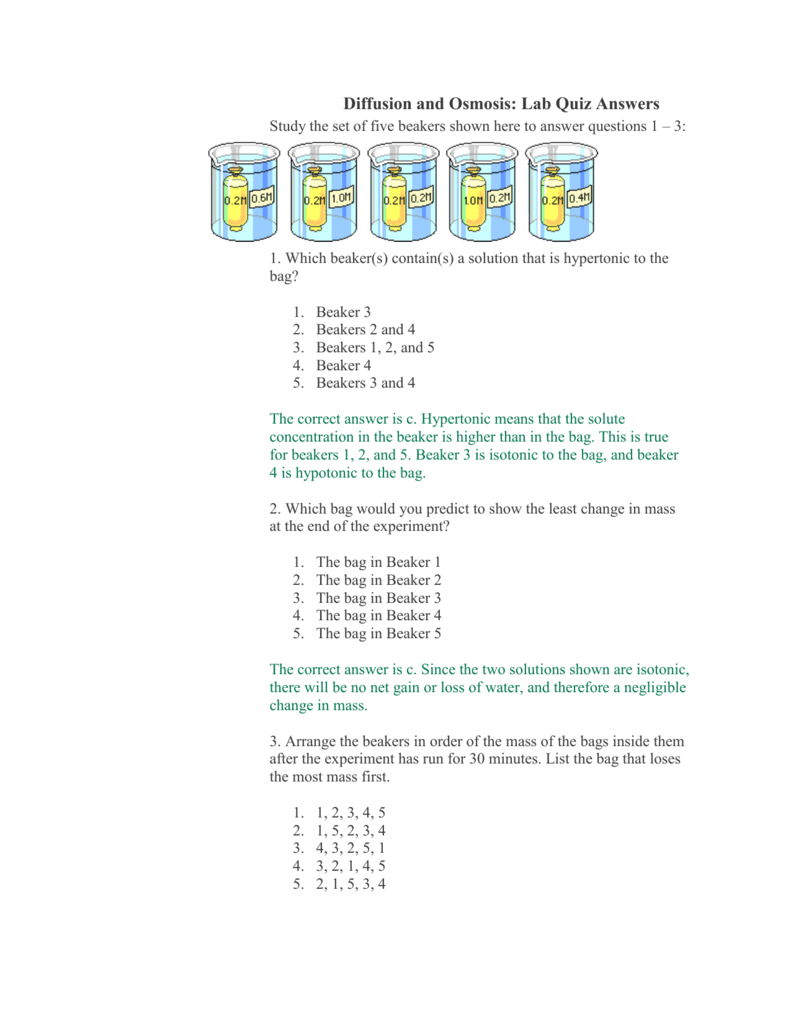
Image: rajuaji6.blogspot.com
Understanding diffusion and osmosis isn’t just an academic exercise; it’s a key to understanding how life works. From the absorption of nutrients in your gut to the regulation of blood volume in your body, these processes are intertwined with every aspect of our existence. This lab is your passport to unlocking the secrets of cellular movement, revealing the invisible forces that shape the very fabric of life.
Delving into Diffusion: The Movement of Molecules
A Molecular Shuffle: Understanding Diffusion
Imagine a crowded room, filled with people chatting and mingling. If you spill a cup of coffee, the scent will gradually spread throughout the room, eventually reaching every corner. This is diffusion in action – the movement of molecules from an area of high concentration to an area of lower concentration. The coffee molecules, initially concentrated at the spill site, spread out until they are evenly distributed throughout the room.
Factors Influencing the Dance: A Closer Look at Diffusion
The rate of diffusion is not a constant but influenced by several factors.
- Temperature: Heat things up, and molecules move faster, increasing the rate of diffusion. Imagine a hot cup of coffee releasing its aroma more quickly than a cold cup.
- Concentration Gradient: The steeper the concentration difference, the faster diffusion occurs. A strong scent of coffee will spread faster than a faint whiff.
- Size and Weight of Molecules: Smaller and lighter molecules move faster than larger and heavier ones. In a crowded room, a small child will navigate more quickly than a large adult.
- Surface Area: The larger the surface area, the faster the diffusion rate. A large room with multiple windows will allow the coffee scent to spread faster than a smaller room with a single window.
- Hypotonic: A solution with a lower concentration of solutes compared to another solution. Think of it as a “dilute” solution with more water molecules than solutes.
- Hypertonic: A solution with a higher concentration of solutes compared to another solution. Picture it as a “concentrated” solution with fewer water molecules and more solutes.
- Isotonic: Two solutions with equal concentrations of solutes. This creates a balanced environment with no net movement of water.
- Cellular Respiration: The process of energy production in our cells relies on the diffusion of oxygen and carbon dioxide across cell membranes.
- Nutrient Absorption: The breakdown of food in our digestive system results in the diffusion of nutrients across the lining of our intestines.
- Waste Removal: Our kidneys filter waste products from our blood, relying on the diffusion of waste molecules across membranes.
- Osmotic Regulation: The balance of water in our blood is maintained through osmosis, ensuring the proper functioning of our organs.
- Drug Delivery: Many medicines are designed to be absorbed into our bodies through diffusion, reaching their target tissues to exert their therapeutic effects.
- Food Preservation: Osmosis is employed in techniques like pickling and salting, where water is drawn out of food, inhibiting bacterial growth.
- Wastewater Treatment: Diffusion and osmosis play a critical role in filtration and purification processes used to treat wastewater, ensuring clean water sources.
- Desalination: Reverse osmosis is used to remove salt from seawater, providing freshwater in areas with limited resources.
- Artificial Membranes: Diffusion and osmosis are exploited in the development of artificial membranes used in dialysis, blood oxygenators, and other medical devices.
In the lab, you’ve likely observed diffusion in action, perhaps using a dye or other substance to track its movement in a solution or through a membrane. These observations help solidify your understanding of the principles at play.

Image: www.studocu.com
Osmosis: Water’s Journey Across Membranes
Water’s Quest for Balance: Understanding Osmosis
Imagine a semi-permeable membrane, like a fine mesh bag. This membrane allows the passage of water molecules but restricts larger molecules. Now, imagine placing this bag in a container filled with water. Water molecules will move across the membrane in a specific direction, driven by the principle of osmosis. Osmosis is the movement of water molecules across a semi-permeable membrane from an area of high water concentration (low solute concentration) to an area of low water concentration (high solute concentration).
From Hypotonic to Hypertonic: The Landscape of Osmosis
To understand the direction of water movement, we need to introduce three key terms:
In the lab, you’ve likely experimented with osmosis using a variety of solutions and observed the changes in their volume. This helps you visualize how water moves in response to differences in solute concentrations. A potato or an egg placed in a hypertonic solution will shrink as water moves out, while in a hypotonic solution, they will swell as water moves in.
Real-World Applications of Diffusion and Osmosis
Diffusion and osmosis are not abstract concepts confined to labs; they are fundamental to life as we know it.
From Cellular Respiration to Drug Delivery: The Intricacies of Life
Our bodies are constantly engaged in diffusion and osmosis.
Beyond the Human Body: The Wider Applications of Diffusion and Osmosis
The principles of diffusion and osmosis extend beyond the realm of human biology. These processes are crucial in various industries and technologies:
Lab 1 Diffusion And Osmosis Answers
Beyond Lab 1: Continuing the Exploration
Your journey into the world of diffusion and osmosis has just begun. As you delve deeper into the complexities of these processes, you will uncover more fascinating applications and intricate details. Explore the impact of different environmental conditions, the role of transport proteins in facilitated diffusion, and the fascinating interplay of diffusion and osmosis in various biological contexts. Your Lab 1 experience has opened a door to a world of scientific discovery, where you can witness the remarkable forces shaping life at its most fundamental level.
Share your observations, delve deeper into the research, and continue to ask questions. The world of diffusion and osmosis, once invisible, is now illuminated by your curiosity and your thirst to understand the world around you. So, embark on this exciting journey and discover the invisible forces that drive the very essence of life.






