Have you ever stared at a complex organic molecule and felt like your brain was about to explode? We’ve all been there. Organic chemistry can be a wild ride, but mastering concepts like the Newman projection can make it a lot smoother. The Newman projection, a simple yet powerful tool, allows us to visualize the spatial arrangement of atoms within a molecule, providing crucial insights into its reactivity and physical properties. In this article, we’ll dive into the world of Newman Projections, exploring practice problems and unraveling the secrets they hold.
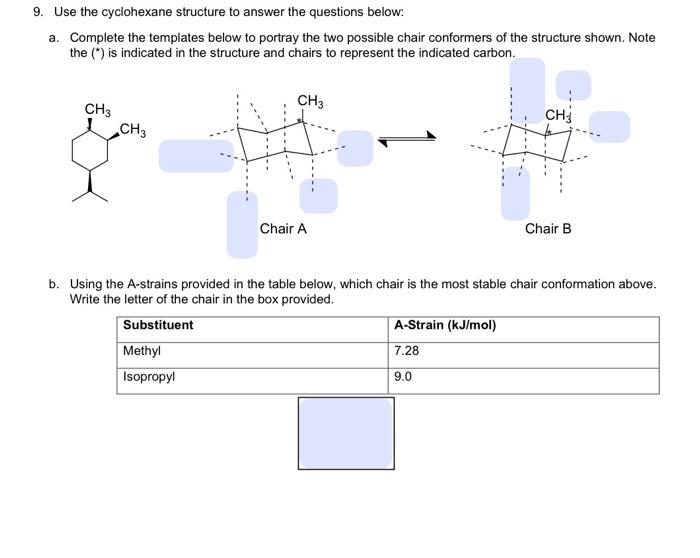
Image: www.chegg.com
The Newman projection, named after Melvin Spencer Newman, is a way of representing a molecule by looking down a specific carbon-carbon bond. It’s like peeking through a keyhole to see the arrangement of atoms on either side of the bond. This representation simplifies the complex three-dimensional structure of molecules, making it easier to analyze steric interactions, understand conformational changes, and predict the outcome of reactions.
Let’s Get Started: A Crash Course in Newman Projections
To truly get a handle on Newman projections, we’ll break down the fundamentals, starting with the basics:
1. Identifying the Central Bond: The Foundation
The first step in creating a Newman projection is to identify the central bond – the bond you’ll be looking down. This is usually a single bond between two carbon atoms. You’ll then be looking at the molecule from the perspective of one carbon atom, with the other carbon atom appearing as a circle in the center of the projection.
2. Front and Back Groups: Looking Through the Keyhole
Imagine yourself looking down the central bond. The groups attached to the front carbon atom are drawn as lines radiating outward from the center circle. These are the “front” groups. The groups attached to the back carbon atom are drawn as lines emanating from the center of the circle, directly behind the front groups. These are the “back” groups.
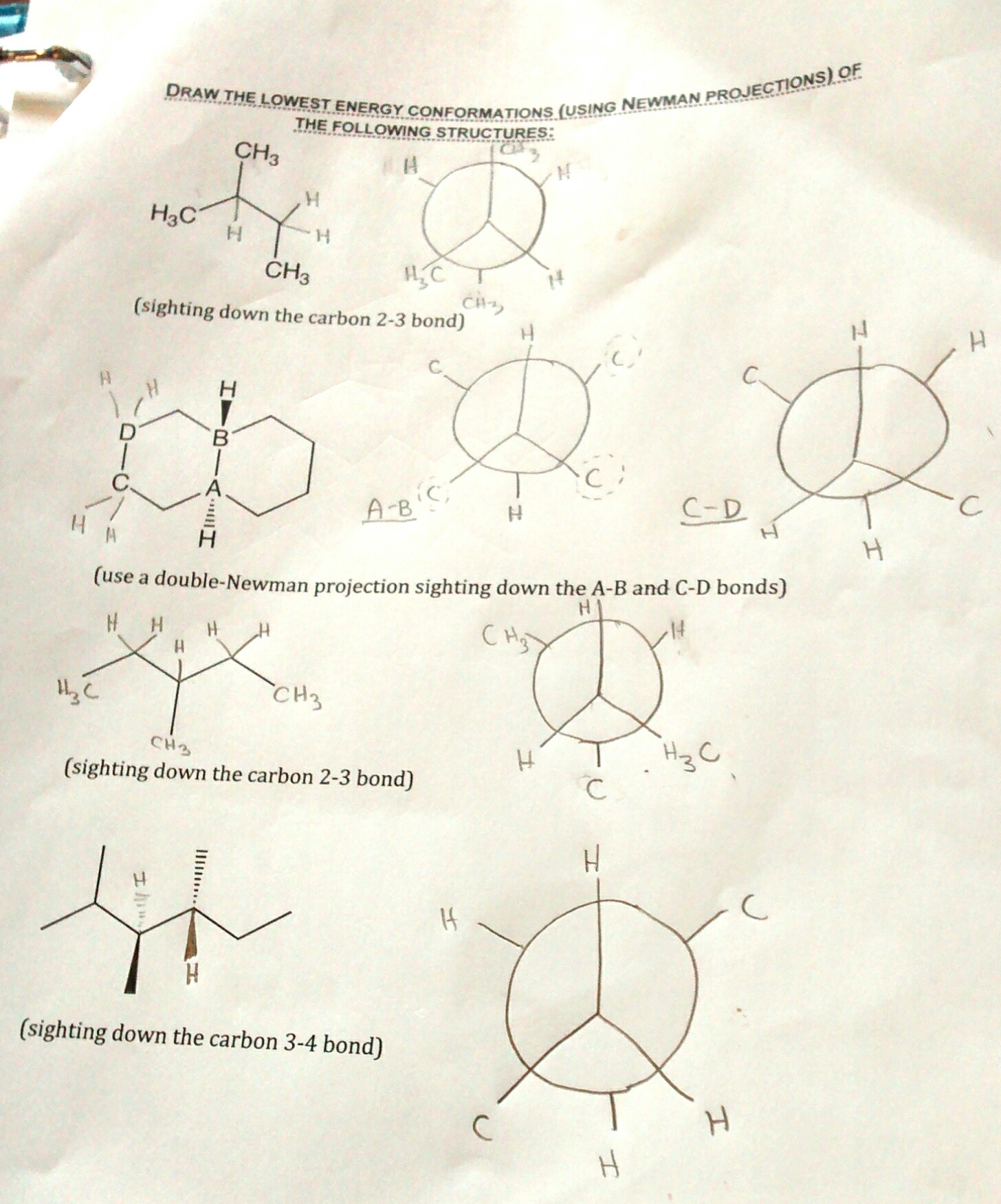
Image: goodimg.co
3. Dihedral Angles: The Key to Conformations
The dihedral angle, also known as the torsion angle, is the angle between the front and back groups. This angle defines the relative position of the groups and plays a crucial role in determining the molecule’s conformation. A dihedral angle of 0° means the groups are eclipsed (directly behind each other), while a dihedral angle of 180° means they are anti (as far apart as possible).
4. Gauche and Anti: Understanding Conformational Relationships
The terms gauche and anti describe the relationship between two substituents on adjacent carbon atoms. A gauche conformation occurs when the dihedral angle between the substituents is approximately 60°, while an anti conformation happens when the dihedral angle is 180°. Gauche conformations typically have higher energy due to steric hindrance (clashing between groups), while anti conformations are generally more stable.
Putting It into Practice: Newman Projection Practice Problems
Now that we’ve laid the groundwork, let’s put our knowledge to the test!
Problem 1: Identifying the Newman Projection
Question: Draw the Newman projection of butane along the C2-C3 bond, with C2 as the front carbon.
Solution:
- Identify the central bond: The C2-C3 bond is our focus.
- Front and Back Groups:
- C2 (front): -CH3 and -H.
- C3 (back): -CH3 and -H.
- Draw the Newman Projection: Draw a circle representing C3 (back) and draw lines extending from the circle to represent the groups attached to C3. Then draw lines for the groups attached to C2, making sure they are aligned with the groups on C3 to show their relative positions.
- Result: The Newman projection of butane along the C2-C3 bond will show two possible conformations:
- Anti conformation: The two methyl groups are on opposite sides (180° dihedral angle).
- Gauche conformation: The two methyl groups are adjacent to each other (60° dihedral angle).
Problem 2: Analyzing Conformations
Question: Which conformation of butane is more stable and why?
Solution: The anti conformation of butane is more stable than the gauche conformation. This is because the methyl groups are further apart in the anti conformation, leading to less steric hindrance or clashing between them. The gauche conformation experiences steric strain, making it higher in energy.
Mastering the Challenges: Newman Projection Practice Problems PDF
Tackling Newman projections requires practice and a good resource like a practice problems PDF. These PDFs typically contain a variety of problems, ranging from simple to complex. This will help you solidify your understanding of Newman projections, develop problem-solving strategies, and build confidence in your ability to analyze and predict the behavior of organic molecules..
Here are some valuable benefits of using Newman projection practice problems PDFs:
- Structured Learning: PDFs provide a clear and organized structure for your practice sessions, allowing you to tackle different types of problems systematically.
- Diverse Problems: These PDFs usually contain a wide range of problems, exposing you to various scenarios and challenging you to think critically.
- Step-by-Step Solutions: Many PDFs offer detailed solutions, allowing you to check your answers and understand the reasoning behind the correct approach.
- Self-Assessment: The practice problems allow you to track your progress and identify areas where you need further practice
- Convenience: Practice problems PDFs are readily available online and can be accessed anytime, anywhere, making learning more flexible.
Where to Find High-Quality Resources:
- Textbooks: Your organic chemistry textbook likely has a practice problems section, including examples using Newman projections.
- Online Resources: Numerous online websites and resources offer free and paid practice problems on Newman projections.
- Study Guides: Study guides specifically designed for organic chemistry often include practice problems for Newman projections.
The Power of Understanding: Using Newman Projections in Chemistry
Learning to master Newman projections is not simply about memorizing rules and drawing diagrams. It unlocks a deeper understanding of organic chemistry, enabling you to:
- Predict Reaction Outcomes: Understanding the conformations and steric interactions of molecules helps predict how molecules will react with each other and the types of products that will form.
- Analyze Molecular Properties: Newman projections provide insights into the physical properties of molecules, such as melting point, boiling point, and solubility.
- Understand Molecular Flexibility: Molecules are not static structures. Newman projections help visualize how molecules can change conformation, giving rise to different reactivity patterns and physical properties.
Newman Projection Practice Problems Pdf With Answers
Conclusion: A Key to Unlocking Organic Chemistry
Newman projections are a powerful tool in organic chemistry. They are essential for analyzing molecular structures, predicting reaction outcomes, and understanding the dynamic nature of molecules. This visualization technique unlocks a deeper understanding of the three-dimensional world of organic molecules. Don’t hesitate to dive into practice problems and make Newman projections your new best friend in the exciting journey of organic chemistry. With practice and a little effort, you’ll be able to master this key skill and unlock the fascinating world of organic molecules.






