The world of chemistry is filled with fascinating processes, and one that particularly caught my attention during my undergraduate years was recrystallization. Remember that moment when you carefully add a tiny seed crystal to a saturated solution, and suddenly, a beautiful, perfectly formed crystal emerges? It’s a process that epitomizes the intricate beauty of chemistry, and the recrystallization of benzoic acid is a classic example. In this blog post, we’ll delve into the practical and theoretical aspects of this process, exploring its significance in purification and providing a step-by-step guide to performing a successful recrystallization experiment.

Image: snso.se
Recrystallization, an essential technique in organic chemistry, plays a crucial role in purifying compounds. It’s not just about getting rid of impurities; it’s about harnessing the principles of solubility and crystal formation to isolate a pure compound, often a solid organic compound, from a mixture. Think of it as a culinary art for chemists – carefully selecting the right solvent and temperature to coax out a desired compound in a beautifully pure form.
Understanding Recrystallization of Benzoic Acid: A Journey into Purity
The Science Behind Recrystallization
Recrystallization is a purification technique based on the principle that the solubility of a solid compound in a liquid solvent is temperature-dependent. Most solids exhibit increased solubility at higher temperatures. When a solution containing impurities and the desired compound is heated, the compound dissolves completely, but the impurities might not. As the solution cools, the compound’s solubility decreases, and it begins to crystallize out. The impurities, being less soluble and potentially more soluble in the cold solvent, will remain in the solution. This separation allows you to isolate the pure compound in the form of crystals.
Benzoic acid is a white, crystalline organic compound with the formula C6H5COOH. It’s a common industrial chemical used in the production of various materials like plastics and dyes. Its purification through recrystallization is a classic example of this technique. Let’s examine the process in more detail.
The Recrystallization Process for Benzoic Acid: A Step-by-Step Guide
- Dissolving the Impure Compound: Start by dissolving the impure benzoic acid in a suitable solvent (typically hot water or a mixture of water and ethanol) to create a saturated solution. The key is to select a solvent in which the desired compound is more soluble at higher temperatures and less soluble at lower temperatures. In the case of benzoic acid, hot water is a good choice because it dissolves benzoic acid well at boiling point but significantly less at room temperature.
- Hot Filtration: To remove any insoluble impurities, filter the hot solution using a hot filtration method. This involves using a heated filter funnel and a preheated filter paper to prevent the compound from crystallizing prematurely in the filter.
- Crystallization: Allow the filtered solution to cool slowly to room temperature to induce crystallization. This process of cooling should be done gradually, ensuring that the crystals have ample time to form. The rate of cooling can affect crystal size, with slower cooling favoring larger, more well-defined crystals.
- Isolation of Crystals: After cooling, the crystals of benzoic acid should be isolated through vacuum filtration. The crystals are collected on a Buchner funnel, and the solvent is drawn off by applying vacuum. This step is crucial because it allows you to remove the remaining solvent and impurities from the crystals.
- Washing and Drying: The collected crystals are washed with a small amount of cold solvent to remove any adhering impurities. The cold solvent minimizes the loss of benzoic acid by dissolving it. Finally, the crystals are dried in a warm oven or under vacuum to remove the remaining solvent.
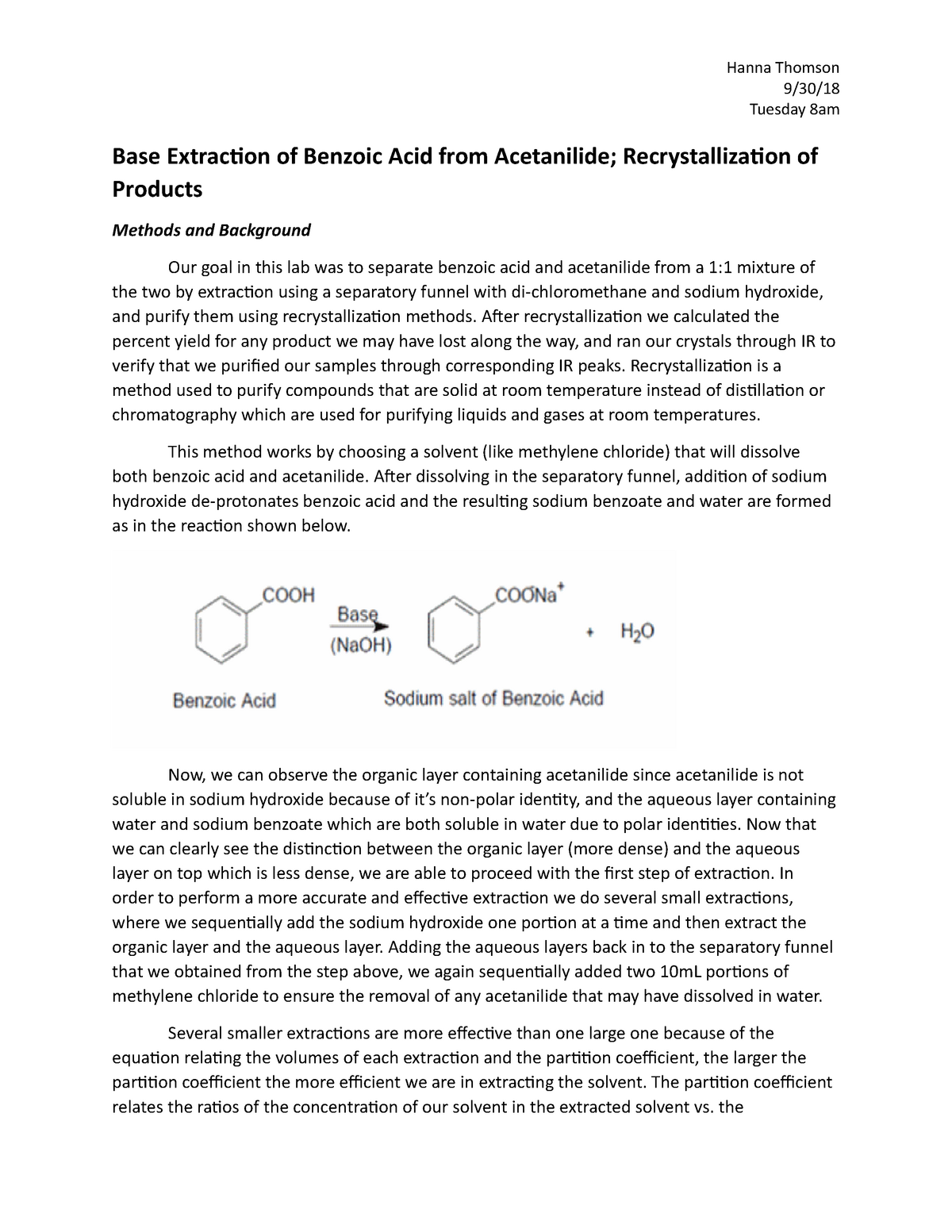
Image: www.studocu.com
Assessing Purity: A Critical Step in Recrystallization
The key to a successful recrystallization is obtaining the purest possible compound. To assess the purity of the recrystallized benzoic acid, several techniques can be utilized. One common method is to determine the melting point of the purified compound. Benzoic acid has a known melting point. You can compare the melting point of your purified benzoic acid to the known melting point. A sharp, narrow melting point range indicates high purity. Another technique is to use thin-layer chromatography (TLC) to compare the purified benzoic acid to the original impure compound. A single spot on the TLC plate confirms the purification.
Latest Trends in Recrystallization
Recrystallization is a time-honored technique, but continuous advancements are happening in materials science and chemical engineering. New techniques and strategies are emerging to refine and optimize the recrystallization process. These innovations include the use of microfluidic devices for controlled crystallization in micro-environments, the development of new solvents and antisolvents for improved solubility control, and the application of high-throughput screening techniques to optimize crystallization conditions.
Researchers are actively investigating methods to minimize energy consumption, reduce time, and enhance the efficiency of recrystallization. For example, using alternative energy sources like ultrasound or microwave irradiation has shown promising results for faster and more energy-efficient recrystallization. This area of research is continuously evolving, bringing about innovative tools and approaches to purify compounds.
Tips for Successful Recrystallization
Here are some tips based on my experience to elevate your recrystallization game:
- Choose the right solvent: The ideal solvent should be one in which the compound is readily soluble at high temperatures and less soluble at low temperatures. The solvent should also be inert, not react with the compound being purified.
- Control the cooling rate: Slow and gradual cooling allows the formation of larger crystals, leading to a more efficient removal of impurities. Rapid cooling can lead to small, impure crystals.
- Use proper filtration techniques: Hot filtration eliminates insoluble impurities. Choose the right filter paper and funnel to prevent clogging and maintain a clear solution during filtration.
- Minimize solvent use: Using an excess of solvent can lead to lower yields and potentially hinder the formation of crystals. Aim for the minimum amount of solvent needed to dissolve the compound completely.
- Consider seed crystals: In some cases, adding a small seed crystal to the solution can initiate crystallization and improve the process.
- Confirm purity: Once the recrystallization is complete, verify the purity of your product using melting point determination and other appropriate methods. The purity of the final product is the ultimate goal.
Following these tips can significantly enhance the efficiency and success of your recrystallization experiments, leading to the purification of your desired compound in a beautiful and pure form.
FAQ: Recrystallization of Benzoic Acid
- Q: What is the purpose of recrystallization?
A: Recrystallization is a purification technique used to remove impurities from solid organic compounds. It relies on the principle of solubility to separate the desired compound from impurities based on their different solubility characteristics.
- Q: How do I choose the right solvent for recrystallization?
A: The ideal solvent should readily dissolve the compound at high temperatures but be less soluble at low temperatures. The solvent should also be inert and not react with the compound being purified. Factors like polarity and boiling point also need to be considered. For benzoic acid, water is a good choice.
- Q: Why is slow cooling important during recrystallization?
A: Slow cooling allows the molecules of the compound to arrange themselves in a more ordered manner, resulting in larger and more pure crystals. Rapid cooling can lead to smaller, less pure crystals, trapping impurities.
- Q: How do I know if my recrystallization was successful?
A: You can assess the success of recrystallization by observing the purity and appearance of the crystals. A pure compound will typically have a well-defined shape, a sharp melting point, and a single spot on a TLC plate.
- Q: How can I improve the yield of my recrystallization?
A: To improve yield, minimize solvent usage, carefully control cooling rates, and utilize appropriate filtration techniques. Also, consider using seed crystals to initiate crystallization.
Lab Report Recrystallization Of Benzoic Acid
Conclusion: Recrystallization – A Journey of Purity for Benzoic Acid
Recrystallization remains a fundamental technique in purification. It is a rewarding process that combines theoretical knowledge with practical skills. This process allows chemists to isolate and purify compounds, leading to advancements in various areas of science and technology. By understanding the principles and following best practices, you can confidently execute this technique and embark on your own journey of purifying benzoic acid!
Are you interested in learning more about recrystallization of benzoic acid or other organic compounds? Share your thoughts and questions in the comments below!






