Remember those late nights hunched over your chemistry textbook, trying to decipher the mysteries of the atom? I certainly do. The diagrams of swirling electrons and dense nuclei were fascinating but felt like a whole new language. However, the journey to understanding atomic structure, particularly the concept of isotopes, becomes much clearer when you have the right guidance, such as the answers to a well-designed worksheet. This article dives into the world of atomic structure and isotopes, demystifying the key concepts and providing you with the tools to understand the building blocks of matter.
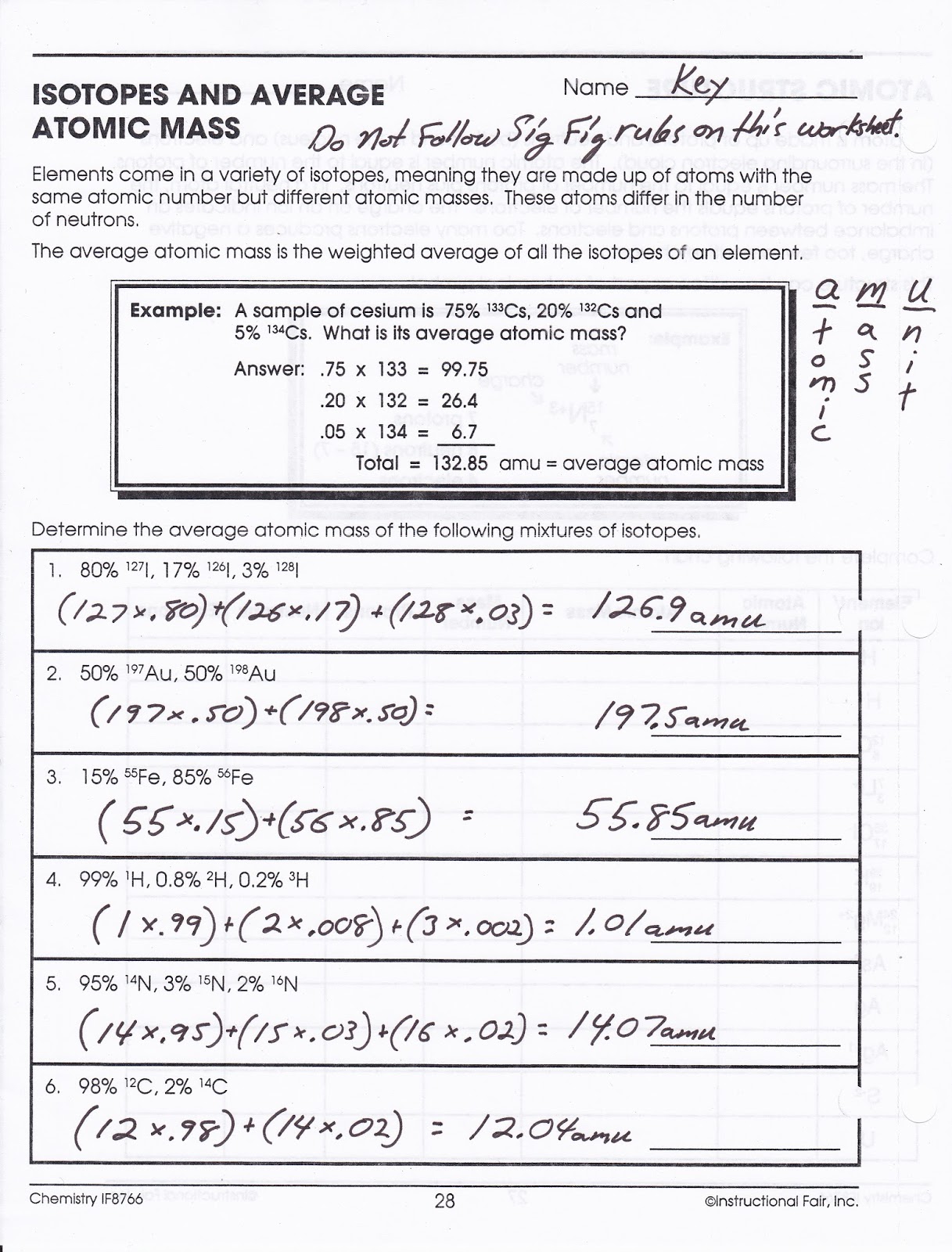
Image: lessonanswermagicemily.z22.web.core.windows.net
The beauty of atomic structure and isotopes lies not just in understanding the fundamentals but also in appreciating the diversity and complexity of the elements that make up our world. From the familiar elements like carbon, oxygen, and hydrogen to the more exotic elements found in the stars, each has its own unique atomic fingerprint, a distinct arrangement of protons, neutrons, and electrons. The journey of comprehending the atom, like any scientific exploration, involves a blend of curiosity, observation, experiment, and understanding the language of the universe.
Delving into the Atom’s Structure: A Visual Journey
Imagine the atom as a miniature solar system. At its center, the nucleus, a tiny, dense core, holds the protons and neutrons. These particles carry the weight of the atom, with protons holding a positive charge and neutrons being neutral. Circling around the nucleus, like planets orbiting the sun, are the electrons, negatively charged particles that are constantly in motion. The arrangement and behavior of these electrons determine the chemical properties of an atom.
Now, let’s delve deeper into the concept of isotopes. Isotopes are atoms of the same element that share the same number of protons (atomic number), but have different numbers of neutrons. This difference in neutrons affects the atomic mass of the isotope. For instance, carbon-12 and carbon-14 are both isotopes of carbon, both with 6 protons. However, carbon-12 has 6 neutrons, while carbon-14 has 8 neutrons, making it heavier.
The Significance of Isotopes
Isotopes are not just theoretical curiosities. They play a vital role in various scientific and technological applications. Radioactive isotopes, like carbon-14, are used for radiocarbon dating, a technique that allows archaeologists to determine the age of ancient artifacts and fossils. In medicine, radioactive isotopes are used in diagnostic imaging techniques like PET scans and in targeted therapies that deliver radiation to specific cancer cells.
The applications of isotopes extend beyond medicine and archaeology. They are essential in various fields, including agriculture, industry, and environmental monitoring.
The Importance of Worksheets in Understanding Atomic Structure
Atomic structure and isotopes worksheets serve as excellent learning tools, offering opportunities to apply your knowledge and visualize these concepts. By tackling various questions, you can build a solid foundation for understanding the principles of atomic structure and the uniqueness of isotopes.
For example, a worksheet might ask you to determine the number of protons, neutrons, and electrons in an atom based on its atomic number and mass number. Or, you might have to identify different isotopes of an element based on their atomic symbols and calculate the average atomic mass of an element.

Image: mrsgingrashths.weebly.com
Mastering Atomic Structure and Isotopes: Tips and Expert Advice
Here are some tips and expert advice to help you master atomic structure and isotopes:
- Visualize the concepts: Drawing diagrams of atoms and isotopes can be immensely helpful. This visual representation helps you understand the spatial arrangement of protons, neutrons, and electrons.
- Use flashcards: Memorizing key definitions, like atomic number, mass number, and isotopes, can be a breeze with flashcards. Create flashcards with the term on one side and its definition or a relevant example on the other.
- Practice, practice, practice: The best way to solidify your understanding of any concept is through practice. Work through as many worksheets, exercises, and problems as possible.
- Seek help when needed: Don’t be afraid to ask for help if you’re struggling with a specific topic. Your teacher, a tutor, or classmates can provide valuable insights and explanations.
Remember, the journey of understanding atomic structure and isotopes is an ongoing one. It’s about building a solid foundation and then continuously adding to it as you encounter more complex ideas in your studies. These concepts are fundamental to chemistry, physics, and other branches of science, so taking the time to master them will open doors to deeper understanding and exciting discoveries.
Frequently Asked Questions (FAQs) About Atomic Structure and Isotopes:
Q: How do I determine the number of protons in an atom?
The number of protons in an atom is equal to its atomic number. This number is unique to each element and is shown on the periodic table.
Q: What is the difference between atomic mass and mass number?
Atomic mass is the average mass of all the isotopes of an element. It is expressed in atomic mass units (amu). Mass number, on the other hand, is the total number of protons and neutrons in the nucleus of an atom.
Q: Why are isotopes important?
Isotopes are important for various reasons. They are used in radiocarbon dating, medical imaging, and treatment, industrial applications, and research in various fields.
Q: What are the different types of isotopes?
Isotopes can be classified into two main types: stable isotopes and radioactive isotopes. Stable isotopes are those that do not decay over time, while radioactive isotopes are unstable and decay over time, emitting radiation.
Q: How are isotopes used in medicine?
Radioactive isotopes are used in medicine for diagnostic imaging techniques like PET scans, which allow doctors to visualize the structure and function of various organs. They are also used in targeted therapies, where radioactive materials are attached to specific molecules that bind to cancer cells and deliver radiation directly to the tumor.
Atomic Structure And Isotopes Worksheet Answers
Conclusion: Embracing the Journey of Atomic Structures and Isotopes
The exciting world of atomic structure and isotopes opens up a whole new perspective on the building blocks of matter. From visualizing the dance of electrons around the nucleus to understanding the diversity of isotopes and their applications, you’ve embarked on a journey of discovery. The key to mastering these concepts lies in a blend of curiosity, active learning, and practice. Keep those worksheets handy, continue to ask questions, and remember that the journey of scientific exploration is filled with endless possibilities.
Are you interested in learning more about atomic structure and isotopes? Share your thoughts and questions in the comments below. We are eager to continue the conversation!






